What is it? Foam or Rubber?
Is it rubber? Is it foam? Or foam rubber. Or rubber foam. Are they really the same…or different? Let’s get to the bottom of this right now.
There are two kinds of rubber: natural rubber and synthetic rubber. The true raw material of natural rubber is Latex from rubber trees. The principal raw material needed to produce synthetic rubber is crude oil. On their own, these two forms are not of much practical use in their initial state.
 Natural rubber from a rubber tree is a liquid form and is commonly referred to as Latex. It consists of thousand of particles that have elastic properties. The particles or monomers (a molecule of one part) must be polymerized (molecules of many parts). That is, the liquid form is converted with the assistance of acid to aid in the coagulation of the particles. Once a solid substance forms, slabs of the latex solid are hung out to dry. After a drying out period there is still a presence of elasticity, but it is not yet dependable (the polymer chains move independently). The next step is to cure the natural rubber to prevent the polymer chains from such freedom. This is called vulcanizing.
Natural rubber from a rubber tree is a liquid form and is commonly referred to as Latex. It consists of thousand of particles that have elastic properties. The particles or monomers (a molecule of one part) must be polymerized (molecules of many parts). That is, the liquid form is converted with the assistance of acid to aid in the coagulation of the particles. Once a solid substance forms, slabs of the latex solid are hung out to dry. After a drying out period there is still a presence of elasticity, but it is not yet dependable (the polymer chains move independently). The next step is to cure the natural rubber to prevent the polymer chains from such freedom. This is called vulcanizing.
Vulcanizing involves sulfur and heat. The natural rubber is cooked. The vulcanization makes the elastic qualities permanent. It can stretch and return to its original form. The additional additives used in vulcanizing makes material harder, stronger, and longer lasting.
Supply and demand often produces innovation and that is true with rubber. During WWII, synthetic rubber was developed and the main supply of rubber trees, at that time, was in Asia. 
Petroleum refineries process crude oil by boiling it, a process known as distillation. During the distillation process, and the miracle of chemistry, various gases are collected. Each gas, or fraction, separates at different levels of heat. Naphtha is one of the fractions.
In the case of man-made rubber, Naphtha is mixed with natural gas to form monomers. If you recall, monomers do not always stick together. Like natural rubber, this material must be polymerized, the converting of monomers to polymers. What’s more, blending multiple monomers is called co-polymerization. These different combinations of monomers create very distinctive types of rubber, each with unique properties. And, yes, the synthetic rubber also must be cured, vulcanized, just like the natural rubber.
That’s a lot of information and it only addresses the raw material! Once the raw material makes it to a manufacturer, it will need to be converted to an end product. This is where the term foam comes in. 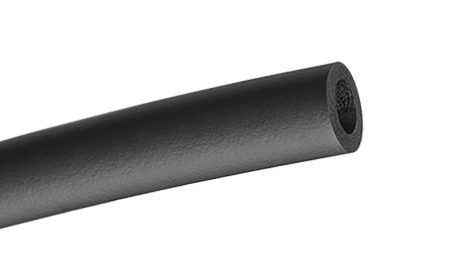 The raw rubber material is transformed from the solid slab of material into a soft, squeezable end product commonly refer to as foam, and in our case, foam grips or foam tubes. Since they are made from rubber, the answer to our original questions above is - all the terms work! It is rubber. It is foam. It is foam rubber and rubber foam.
The raw rubber material is transformed from the solid slab of material into a soft, squeezable end product commonly refer to as foam, and in our case, foam grips or foam tubes. Since they are made from rubber, the answer to our original questions above is - all the terms work! It is rubber. It is foam. It is foam rubber and rubber foam.
The only thing left to do is decide which of the monomer combinations will best serve your requirements. As noted above, depending on the combinations, each distinctive type of rubber has unique properties. One of the most popular types of rubber is Ethylene Propylene Diene Monomer (EPDM). Its primary trait is its ability to withstand excessive outdoor elements. Nitrile Polyethylene Vinyl Chloride or NPVC is an economical alternative, however, it is not considered best for outdoor environments.
Follow the links on this page to navigate to more details on the various materials we use for our grips and tubes.
Read about our Vinyl Material. Or is it Plastic?.
Now, about that closed cell question. (More to come).




